Respiratory syncytial virus (RSV) is a very contagious virus that causes a respiratory tract infection, affecting 64 million people per year worldwide. Currently, there is no licensed vaccine to prevent RSV infections. The only FDA approved treatment option is a humanized monoclonal antibody (IgG) against the RSV fusion protein, known as palivizumab. Creative Diagnostics has released a range of RSV recombinant antigens and antibodies. All of them are produced using a standardized production process to ensure the highest quality. These reagents can assist your RSV for vaccine development.
Performance
- Suitable for a range of applications, including ELISA, IF and WB
- Specific to RSV, no cross reaction with influenza A, influenza B, and adenoviruses
Background
Respiratory Syncytial Virus (RSV) belongs to paramyxoviridae family. RSV is an enveloped RNA virus with a non-segmented single-stranded negative-sense genome. The disease caused by RSV is called syncytial virus pneumonia. This disease is a common interstitial pneumonia in children, often occurred in infants and young children. RSV has two antigenic subgroups: A and B, their genome encodes ten proteins, including seven structural proteins (G, F, M1, M2, P, L, N) and three nonstructural proteins (NS1, NS2, SH). F and G proteins are the two major surface proteins that control viral attachment and the initial stages of infection. F protein, also known as fusion protein, fuses the envelope and the host cell into syncytia and induce humoral immunity and cellular immunity. G protein, also known as adhesion protein, allows the virus to adsorb on the cell surface of the host, mediated RSV and cell contact, which leads to the infection. Both F and G proteins can stimulate the body to produce neutralizing antibodies during natural infection, they are the most important protective antigens.
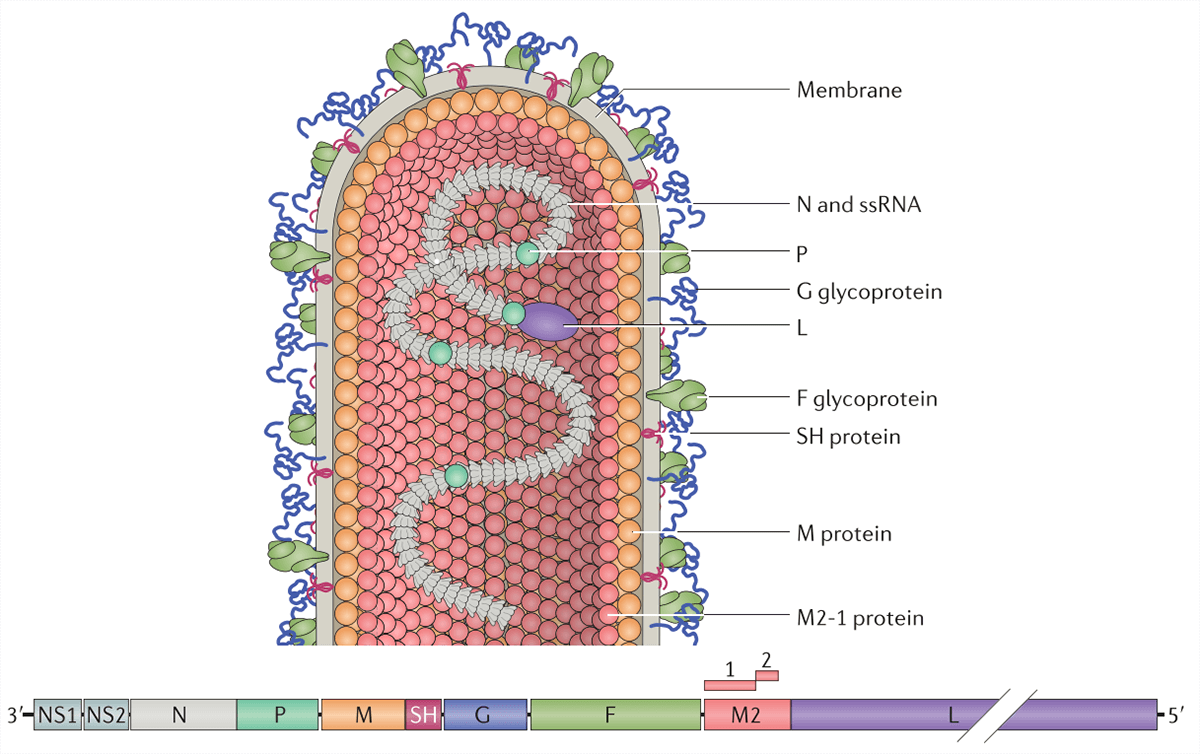 Fig. 1 Respiratory syncytial virus virion (Battles MB & McLellan JS. 2019)
Fig. 1 Respiratory syncytial virus virion (Battles MB & McLellan JS. 2019)
Currently, there is no vaccine approved for RSV. However, many promising vaccine candidates are in clinical trials or preclinical development comprising six different vaccine modalities each targeting one or more of the three major target populations. Four candidates, GSK, Janssen, Moderna and Pfizer, are in late-stage clinical trials. Once approved, an RSV vaccine will be most useful for children and seniors.
References
- Soto JA, Stephens LM, Waldstein KA, et al. (2020). Current Insights in the Development of Efficacious Vaccines Against RSV. Frontiers in Immunology. 11.
- Battles MB & McLellan JS. (2019). Respiratory syncytial virus entry and how to block it. Nature Reviews Microbiology. 17, 233-245.
- Falsey AR, Hennessey PA, Formica MA, et al. (2005). Respiratory syncytial virus infection in elderly and high-risk adults. The New England Journal of Medicine. 352(17):1749-1759.
- Kendall P. (2021). The race to make vaccines for a dangerous respiratory virus. Nature. 600, 379-380.






