In molecular biology, the words extrinsic pathway may refer to both the extrinsic pathway of apoptosis and the extrinsic pathway of blood coagulation. Here we mainly talk about the first kind of extrinsic pathway which is extrinsic apoptosis pathway. Extrinsic apoptosis pathway is one of the signal pathways which may trigger the process of programmed cell death namely cell apoptosis.
Overview of Extrinsic Apoptosis Pathway
Apoptosis is known as a physiological process of cell deletion and is also a process of programmed cell death, resulting in morphological change and DNA fragmentation. It is stimulated by external or internal events of cells, one of which is the extrinsic pathway mediated by the death receptor. The death receptors include Fas receptors, tumor necrosis factor (TNF) receptors, and TNF-related apoptosis-inducing ligand (TRAIL) receptors. As a surface receptor, for example, TNF receptor-1 (TNF-R1), it will interact with TNF to induce the recruitment of adaptor proteins such as Fas-associated protein with death domain (FADD) and Tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD), which recruits a series of downstream factors, including Caspase-8, which is a critical mediator of the extrinsic pathway, resulting eventually in cell apoptosis.
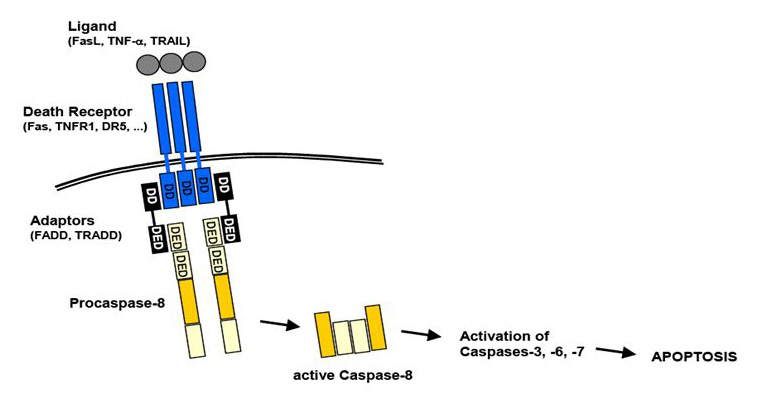 Figure 1. An overview of extrinsic apoptosis pathway.
Figure 1. An overview of extrinsic apoptosis pathway.
Process and Regulation of Extrinsic Apoptosis Pathway
The extrinsic pathway that initiates apoptosis is triggered by a death ligand binding to a death receptor, such as TNF-α to TNFR1. The TNFR family is a large family consisting of 29 transmembrane receptor proteins, organized in homotrimers and activated by binding of the respective ligand(s). They share similar cysteine-rich extracellular domains and have a cytoplasmic domain of about 80 amino acids called the “death domain” (DD). This death domain plays a critical role in transmitting the death signal from the cell surface to the intracellular signaling pathways. There are 19 members of the TNF ligand family and binding may result in a number of responses, including proliferation, inflammation, and apoptosis, depending on the adaptor proteins associated with the activated receptor.
TNFR may also stimulate pro-inflammatory pathways leading to activation of NFκB, via recruitment of RIP. The death domain kinase RIP is essential for TRAIL-induced IκB kinase (IKK) activation. It has been identified that the binding of TNF-α and TNFR1 activates NFkB pathway, which favored both cell survival and apoptosis, depending on the cell type and biological context.
Besides TNFR1, the Fas and DR4/DR5 also involved the pathway as death receptors and bind CD95 and TRAIL, respectively. All of the ligand binding to receptors will lead, with the help of the adapter proteins (FADD/ TRADD) to recruitment, dimerization, and activation of a caspase cascade and eventually cleavage of both cytoplasmic and nuclear substrates. To date, the best-characterized ligands and corresponding death receptors include CD95/Fas, TNF-α/TNFR1, Apo2L/DR4 and Apo2L/DR5.
Receptor trimerization results in recruitment of several death domains and eventually recruitment and activation of caspase-8 and caspase-10. Active caspase-8 and caspase-10 then either initiate apoptosis directly by cleaving and thereby activating executioner caspase-3/6/7), or activates the intrinsic apoptotic pathway through cleavage of the BID to induce efficient cell death. Immuno-blot analysis also revealed that the caspase-6 inhibitor blocked the cleavage of lamin A/C, whilst the caspase-3/7 inhibitor blocked the cleavage of poly (ADP-ribose) polymerase (PARP). Activation of caspase-8 may be prevented by FLICE inhibitory protein (FLIP).
Taken together, these results suggest that activation of caspases, the subsequent cleavage of lamin A/C and PARP, and the NFkB pathway are involved in the extrinsic pathway of cell apoptosis.
Importance of Extrinsic Apoptosis Pathway
Apoptosis is a process whereby cells undergo programmed death and is a counterbalance to proliferation. It is morphologically distinct from necrosis and involves shrinkage and fragmentation of both the nucleus and the cell without rupture of the cellular membrane. This prevents inflammation of the surrounding tissue. Apoptosis relies on activation of distinct signaling pathways that are often deregulated in cancer. Thus, our ability to exploit these pathways to design more effective and non-toxic therapies for cancer is dependent on our understanding of the biologic mechanisms.
References:
- Elmore, S. Apoptosis: a review of programmed cell death. Toxicology Pathology. 2007, 35(4): 495-516.
- Krakstad, C; Chekenya, M. Survival signaling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Molecular Cancer. 2010, 9: 135.







